출처 : www.corrosion-doctors.org/FuelCell/sofc.htm
Solid Oxide Fuel Cells
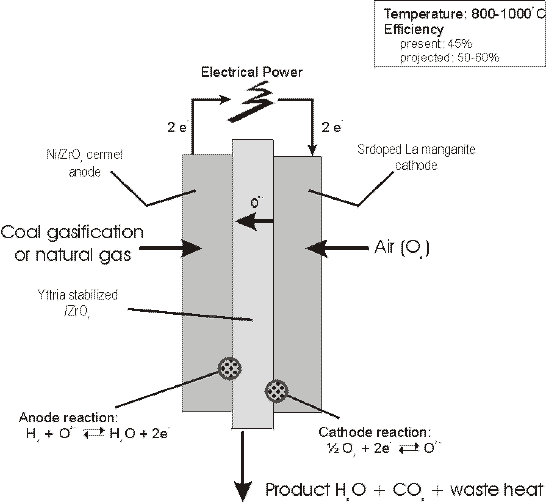
SOFCs have recently emerged as a serious high temperature fuel cell technology. Of primary importance is the fact that SOFCs require no liquid electrolyte, with associated Corrosionand electrolyte management problems. This system is based upon the use of a solid ceramic as the electrolyte and operates at extremely high temperatures (1000°C). This high operating temperature allows internal reforming, promotes rapid electrocatalysis with non-precious metals, and produces high quality byproduct heat for cogeneration. It is best suited for provision of power in utility applications due to the significant time required to reach operating temperatures. Programs are underway in Japan and in U.S. The development of suitable materials and the fabrication of ceramic structures are presently the key technical challenges facing SOFCs. A schematic description of the components in a SOFC is shown here:
The electrolyte typically consists of a solid non-porous such as Y2O3 stabilized ZrO2 with conductivity based on oxygen ions (O2-). Typically the anode is made of a Co-ZrO2 or Ni-ZrO2 cermet, and the cathode of Sr doped LaMnO3. The solid state character of all SOFC components means that there is no fundamental restriction on the cell configuration. Cells are being constructed in two main configurations, i.e. tubular cells, such as those being developed at Westinghouse Electric Corporation since the late 1950s, and a flat plate configuration adopted more recently by many other developers.
EPRI considers SOFCs, which employ a ceramic, solid-state electrolyte (zirconium oxide stabilized with yttrium oxide), the only fuel cell technology with the potential to span market-competitive applications from residential loads as small as 2 kW to wholesale distributed generation units of 10 - 25 MW. Because SOFCs operate at a higher temperature than MCFCs, their simple system efficiency is theoretically not quite as good as that of MCFCs, although it is better than the efficiencies of PAFCs and PEM fuel cells. But the 850 - 1000°C waste heat that SOFCs produce, when used for cogeneration or for driving an integrated gas turbine, can boost overall system energy efficiency to very attractive levels. Moreover, SOFCs operate at a high enough temperature to incorporate in their an internal fuel reformer that uses heat from the fuel cell, along with recycled steam and a catalyst, to convert natural gas directly into a hydrogen-rich fuel.
High-efficiency systems coupling advanced SOFCs with small gas turbines and having a combined rating in the range of 250 kW to 25 MW are expected to fit into grid-support or industrial on-site generation markets, and they potentially could compete head-on with wholesale power rates. Both PEM fuel cells and SOFCs could someday be suitable for small-scale residential market applications if ultimate cost goals are reached, i.e. $1000/kW.